- Electronegative Atom
- Electronegative Atoms In Periodic Table
- Electronegativity Of As
- Electronegative Atom Of Oxygen And Calcium
Structure & Reactivity
An electronegative atom pulls valence electrons away from the atom and effectively decreases the electron density around the nuclei. Thus, a lower value of B0 is. In general electronegativity is the measure of an atom's ability to attract electrons to itself in a covalent bond. Because fluorine is the most electronegative element, the electrons tend to 'hang out' more toward the fluorine atom when fluorine is covalently bonded to other atoms. Oxygen is the 2nd most electronegative element.
Nuclear Magnetic Resonance Spectroscopy
NMR5. Factors in Chemical Shift: Electronegativity
Electronegativity is a second factor that influences NMR spectra. The frequency of radio waves absorbed by an atom depends on the magnetic field experienced at the nucleus. The magnetic field experienced at the nucleus depends on the amount of electron density around the atom. Consequently:
- the more electron density present, the further upfield the shift in the spectrum.
- the less electron density present around the atom, the further downfield the shift.
Tributylamine has an NMR spectrum with four peaks, one for each inequivalent carbon in the structure. These peaks are spread out just a little bit more than in a hydrocarbon; there are more peaks showing up further downfield. The carbon next to the nitrogen is the one that shows up furthest downfield.
Figure NMR7.13C NMR spectrum of tributylamine.

Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
- usually, a tetrahedral carbon shows up in the upfield half of the spectrum.
- the region from about 0 to 100 ppm can be thought of as the sp3 window.
- an electronegative atom moves a peak further downfield within the sp3 window.
Dibutyl ether has peaks that show up even further downfield. As in tributylamine, the carbon next to the heteroatom, in this case an oxygen, shows up the furthest downfield. The other carbons in the chain also show up a little farther downfield than they would in butane, but the further from the oxygen they are, the less effect the oxygen has on them. This is a typical inductive effect. In an inductive effect, atoms have an effect on each other through sigma bonds, but the further apart the atoms are the smaller the effect.
Figure NMR8.13C NMR spectrum of butyl ether.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
Notice that the absorbance of a carbon next to an oxygen atom was shifted even more to the left than a carbon next to a nitrogen atom. The more electronegative the neighbour, the greater the effect. You would probably expect a less electronegative neighbour than carbon will result in a shift to the right, and generally that's the case.
In summary:
- electronegative elements draw attached carbons downfield.
- the more electronegative the element, the farther downfield the attached carbon.
- electronegative elements also have an effect on atoms further down the chain, drawing them downfield.
- the farther the atom is from the electronegative atom, the smaller the effect.
- the effect of electronegative atoms on their neighbours is called an inductive effect.
Methane (CH4) absorbs at about 5 ppm in the 13C NMR spectrum. Chloromethane absorbs at about 30 ppm. Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar.
Dichloromethane or methylene chloride (CH2Cl2) shows up at about 55 ppm, and trichloromethane or chloroform (CHCl3) at about 80 ppm. The more bonds there are to an electronegative element, the further downfield the carbon absorbs. In the case of a chlorinated carbon, each additional chlorine moves the peak about 25 ppm further downfield.
- the effect of electronegativity is additive.
- the more electronegative elements attached to a carbon, the farther downfield it absorbs.
These trends are also seen among sp2 carbons. An oxygen atom attached to an sp2 carbon results in a downfield shift, to about 160 ppm. On the other hand, if a trigonal planar carbon is double bonded to an oxygen atom, the shift can be much farther; it actually ranges from 160 to 210 ppm, depending on what else is attached to the carbon, and is most commonly seen around 180 ppm.
Electronegative Atom
Problem NMR5.1.
Suggest an assignment for the following 13C NMR peaks:
a) 12 ppm b) 58 ppm c) 22 ppm d) 41 ppm
Problem NMR5.2.
Electronegative Atoms In Periodic Table
Draw the predicted 13C NMR spectra for the following compounds.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
/PeriodicTableElectronegativity-56a12a045f9b58b7d0bca77c.jpg)
Navigation:
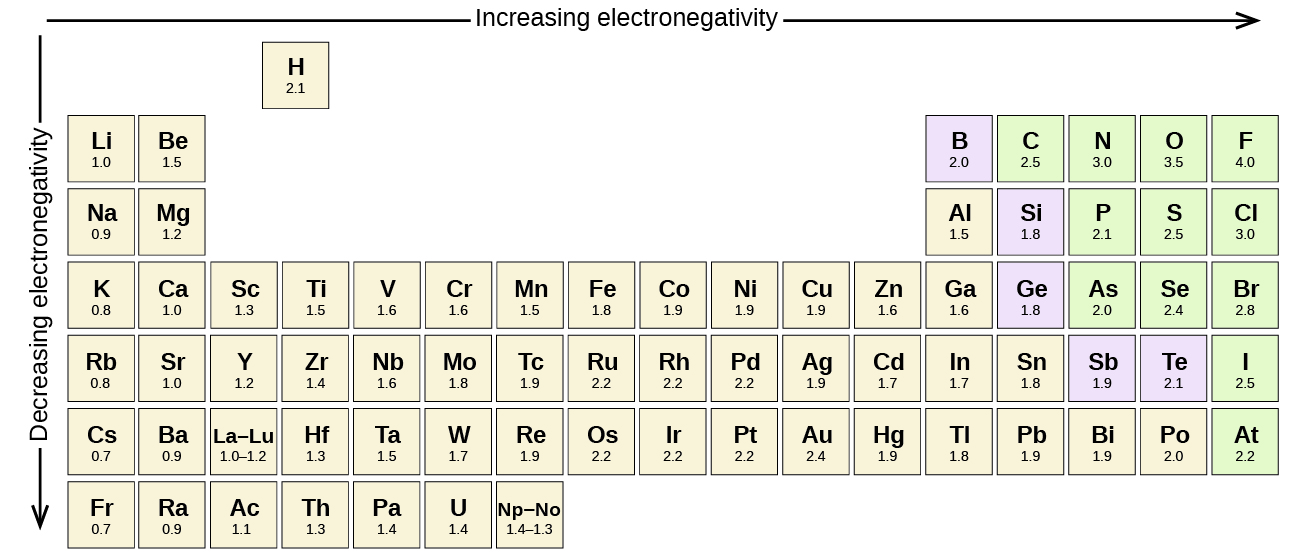
Consider a simple molecular bond such as that in carbon monoxide (CO). This molecule has 2 nuclei and 6+8 = 14 electrons around these nuclei. The usual Lewis electron-dot structure for CO is (recall that the Lewis structure contains only the valence electrons): Since a single carbon atom has 6 electrons around it and a single oxygen atom has 8 electrons around it, one might expect that in the CO molecule carbon has 6 electrons around it and oxygen has 8 electrons around it. However, that does not turn out to be the case. It turns out that oxygen is slightly better than carbon at 'pulling' electrons towards it. Therefore in the CO molecule O has slightly more than 8 electrons around it (giving it a net negative charge) and C (being robbed of this electronic charge by O) has slightly less than 6 electrons giving it a slightly positive charge (equal but opposite to that of the oxygen). The net result is a molecular bond that is electrically 'polarized'. e.g., ,where the Greek 'delta' symbols represent slight positive and negative charges. The ability of an atom in a covalent bond to pull electrons towards it is called its 'electronegativity'. Atoms can be assigned electronegativities. Generally, the higher the electronegativity the more electronegative the atom. As a general rule, the electronegativities of atoms increase from bottom to top and from left to right (not including the noble gases). Consequently Fluorine is the most electronegative element. Specific electronegativities can be found using the periodic table link at the top of this and every other page in this website. All molecular bonds between different atoms are polar. Bonds between the same type of atom (e.g., C-C) are not polar since they both have the same electronegativity. The fact that bond can be polar explains a wide variety of physical and chemical phenomena and will be discussed at the appropriate junctures in these notes. |
Electronegativity Of As
Back to index
Electronegative Atom Of Oxygen And Calcium
